How Many Molecules Are in 5 Mg of Aspartame
The number of S atoms are present in 550 mg of allicin is 4110 19 S atoms. How many atoms are in 5 mg of allicin.

How Many Molecules Are In 5 Mg Of Aspartame Brainly Com
Also molar mass of aspartame 2943 grams.
. How many atoms of nitrogen are in 12 g of aspartame. Aspartame has the chemical formula C14H18N2O5 and its molar mass is calculated to be 29430 gmol. To calculate the number of moles we use the equation.
How many moles of molecules are present in 100 g aspartame. Molar mass 1201 gmol 14 1008 gmol 18 1401 gmol 2 1600 gmol 5 molar mass 2943 gmol. 170 10 5 m o l 6022 10 23 m o l e c u l e s 1 m o l.
102 x 1019 e How many atoms of nitrogen are in 12 grams of aspartame. Calculate the number of moles of aspartame. Să convertim masa dată 5 mg la.
N atoms 102. One may also ask how do I calculate moles. Using the Avogadros constant we need to convert the values into molecules.
What is the molar mass of aspartame. How many atoms are in 5 mg of allicin. What is the mass of one mole of aspartame having formula c14h18n2o5.
Mass of aspartame 5 mg 0005 grams. Putting values in above equation we get. Up to 256 cash back How many molecules are in 5 mg of aspartame.
Were asked to find the number of molecules of a given mass of a substance. How many molecules are in 50 mg aspartame. Aspartamul are formula chimică C _14 H _18 N _2 O 5 iar masa molară se calculează a fi 29430 g mol.
1 g 1000 mg Molar mass of aspartame 2943 gmol. So there would be moles of aspartame in 100 mg of it. One may also ask how many molecules are in 5g of aspartame.
What is the molar mass of aspartame. Considering this how many moles are in 5 mg of aspartame. Aspartame is and its molar mass is 29431 grams per mole.
According to mole concept. Mass of aspartame 5 mg 0005 grams. Given mass of aspartame 200 mg 0002 g Conversion factor.
The number of S atoms are present in 550 mg of allicin is 4110 19 S atoms. Using Avogadros number there are 1023 E19 molecules each. A mole is a unit measurement for the amount of substance in an international system of units ie SI unit.
Calculate the molar mass of aspartame. Calculate the molar mass of aspartame. Share this link with a friend.
1000 How many atoms of nitrogen are In 12 g of aspartame. Now moles of aspartame eqfracmassmolar Mass of aspartame 5 mg 0005 grams. How many moles of aspartame are present in 500 mg of aspartame.
Solution- It is a unit conversion problem where we are asked to convert mg of aspartame to moles. Now moles of aspartame eqfracmassmolar Mass of aspartame 5 mg 0005 grams. Now moles of aspartame eqfracmassmolar Mass of aspartame 5 mg 0005 grams.
To calculate the number of moles we use the equation. 5mg 1g 103mg 1lmol 29430g 170 105 mol aspartame. 50 𝑔 𝑎 𝑎 𝑎 1 𝑔 1000 𝑔 1 294304 𝑔 6022 1023 1 1 1019 e.
1 g 1000 mg Molar mass of aspartame 2943 gmol. Also Know how do I calculate moles. 29434 g 1 mole.
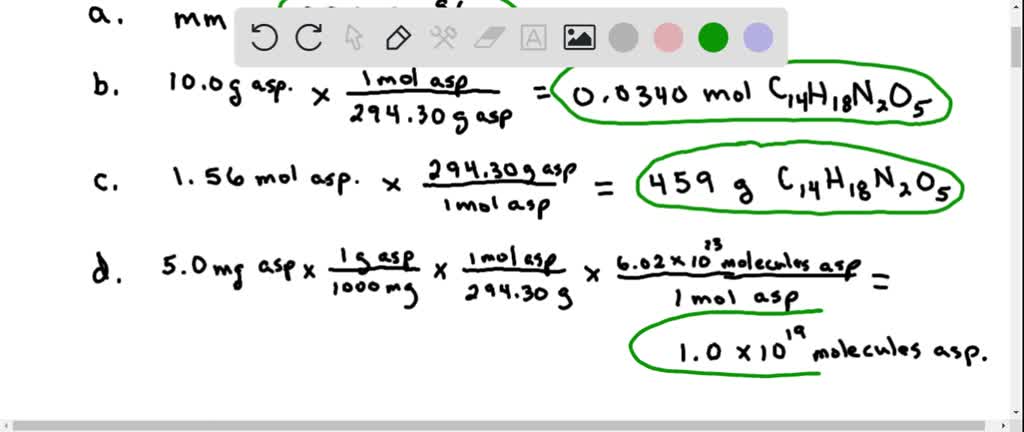
6 of 6 Review Constants Periodic Table How many molecules of aspartame are present in 500 mg of aspartame. Calculate the mass in grams of 156 mol aspartame. It is marketed by GD.
Musashixjubeio0 and 14 more users found this answer helpful. The molecular formula of aspartame is C14H18N2O5. Considering this how many moles are in 5 mg of aspartame.
Start with the number of grams of each element given in the problem. The molar weight is 2943 gramsmol so there are 00001699 moles of aspartame. Also molar mass of aspartame 2943 grams.
1 xx 10 19 molecule C _14 H _18 N _2 O _5 S-a cerut să găsim numărul de molecule dintr-o anumită masă a unei substanțe. Answer- There are moles. The number of molecules in given amount of aspartame is.
What is the mass of one mole of aspartame having formula c14h18n2o5. 100 x 10 asp c. The molecular formula of aspartame is 18 a.
Express your answer using three significant figures. 1 mole of a compound contains number of molecules. Putem face acest lucru folosind masa molară a substanței și constanta lui Avogadro 6022 xx 10 23.
Given mass of aspartame 200 mg 0002 g Conversion factor. Start with the number of grams of each element given in the problem. 12 𝑔 1 294304 𝑔.
Lets convert the given mass 5 mg to moles of aspartame. 170 105 mol 6022 1023lmolecules 1mol 1. It is marketed as Nutrasweet.
Now that we have grams I can divide by the molar mass. How many molecules are in 50 mg of aspartame. Putting values in above equation we get.
Mg are converted to grams and then the grams are converted to moles as. According to mole concept. 1 10 19 m o l e c u l e s.
We can do this using the molar mass of the substance and Avogadros constant 60221023. The chemical formula for Aspartame is C14H18N2O5. A Calculate the gram-formula-mass of aspartame.
49 x 1021 atoms of nitrogen. Searle as Nutra Sweet. D How many molecules are in 5 mg of aspartame.
In one mole of aspartame theres 29430 g. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose table sugar when dissolved in water. Using Avogadros constant we can convert this value to molecules as so.
Express your answer using four significant figures. Up to 24 cash back 6022 x 1023 molecules N2.

11 Foods To Avoid With Pcos The Ultimate Guide Types Of Diabetes Alternative Sweeteners Diabetes Diet Plan
Solved Aspartame Is An Artificial Sweetener That Is 160 Times Sweeter Than Sucrose Table Sugar When Dissolved In Water It Is Marketed As Nutrasweet The Molecular Formula Of Aspartame Is Mathrm C 14 Mathrm H 18 Mathrm N 2
No comments for "How Many Molecules Are in 5 Mg of Aspartame"
Post a Comment